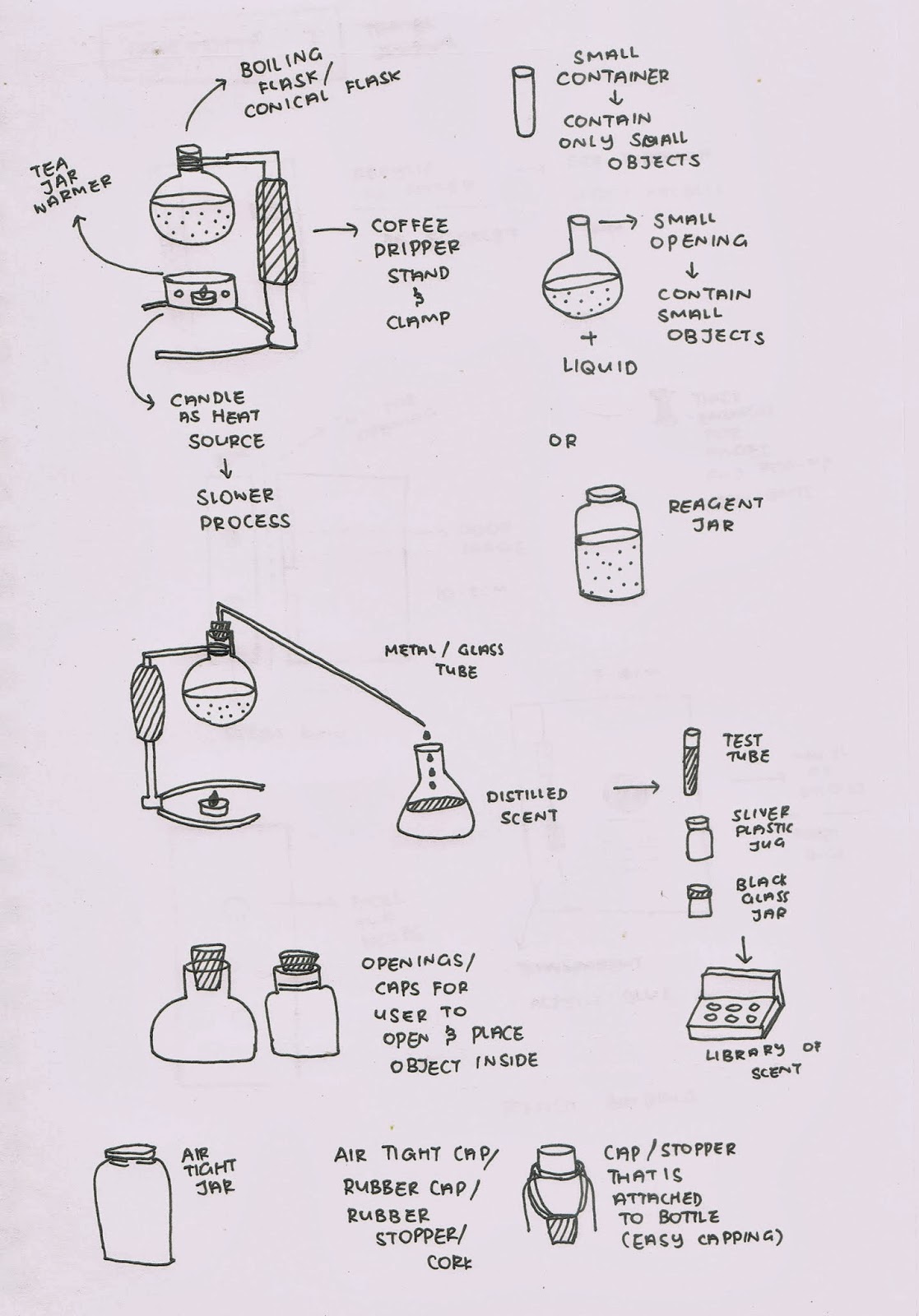
After planning out the steps and things needed for the first experiment, I made a trip down to Art friend and see what materials I can get to build the set up for the distiller. I got some cork stoppers and a acrylic hollow tube and did some DIY since I have not gotten hold of the professional apparatus.
I drilled a hole into the cork stopper which was quite difficult because the drill is too strong for the cork and it might break if too much force is applied on it. Also, it was hard to cut the acrylic tube and when they break into two pieces, the ends are not sharp and in a weird angle and shape.
The few things needed before I started out with the distillation experiment. I used some dried lavender flowers to test out because they have quite a strong smell and I would just like to try if this glassware I bought would work. I also prepared a candle and candle holder to be the supporting heat source. Also, an extra regeant jar at the end of the hollow tube to collect the liquid. And I began with the experiment.
Firstly, I placed a spoonful of lavender flowers into the right smaller boiling flask shape inside. And I capped it back with the cork stopper what is attached to the hollow tube so that no gas would escape.
After that, I added boiling hot water into the left opening of the glassware, which will fill up and surround the inner glass compartment and heat up the lavender flowers and create vapour. And I quickly covered the opening with a rubber stopper so that heat will not escape.
I then lighted up the candle and placed into the candle holder, and placed the flask onto the candle holder. The candle here will be a supporting heat source because the boiling water in the flask will surely decrease in temperature as it is exposed to the room temperature. However, the flame keep extinguishing after a few tries. Which I realised that the candle holder has no opening for air to pass through hence no oxygen and the candle keeps extinguishing. Its good to realise it now through my mistakes.
The experiment did not work as the water was not a a boiling temperature which is not hot enough to produce vapour. So I moved the whole set up to the stove. I poured out most of the water inside so that it will not take so long to boil. The water droplets on the top of the surface was really formed so much faster. They were formed not only in the inner flask but also on the outer flask.

During the process, I turned up the fire so that it would not take so long. I forgot about the pressure and the rubber stopper on the left actually flew out and hit the ceiling. Thank goodness the glass did not explode although it was heat resistant. Also it was quite dangerous, I might need to wear googles next time doing my experiments that includes fire. So I continued to boil the flask but this time with the rubber stopper taken away, steam was constantly running out through the left opening and the top of the inner glass gathered plenty of vapour drops. It crept out into the hollow tube but did not come out and into the jar.
The reason was because the material of the hollow tube is not a surface that is cold enough to allow the vapour to change into liquid state. Moreover, the angle was too sharp and not tilted thats why it was hard for the water collected to flow downwards.
At the end, I managed to collect the scent but not in the correct way. As seen in the small bottle above, the colour of the liquid is wrong. Because the liquid should be colourless and pure. This experiment and set up was not a success but I guess at least now I know that this method is strike out the list and more can be improved.
Tomorrow, I will be checking with a supplier about the stocks for the real chemistry glasswares and equipments. So that I can start on another experiment. I guess sometimes it is alright to fail.
















































.jpg)



