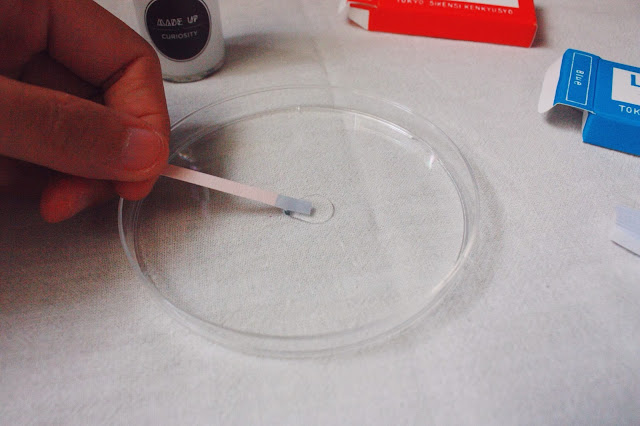
Today I decided to take the litmus papers in red and blue out to experiment, I bought them in Tokyo during the previous trip. Although I know that the bottled solutions contains acid and alkali, I just wanted to test them out with the litmus paper and see how it works and if they work accurately because the chemicals were DIY-ed. I also tried to pack them into the small test tubes in case I want to implement the usage of the litmus paper for the outcome 1, and they will also fall into the chemistry apparatus theme.
Before making the experiment, I went online to search for information and study about the litmus paper. So this is how it works :
1. If the blue litmus paper turns red where you have tested it, the chemical is an acid. If there is no reaction then it is not and you can move on to testing the chemical with the red litmus paper.
2. If the red litmus paper turns blue where you have tested it, the chemical is a base. If there is no reaction to either the red paper or the blue paper, then the substance has a neutral Ph and is neither an acid nor a base.
3. There is also a purple, or neutral litmus paper. This paper turns red in the presence of an acid or blue in the presence of a base.
The first dip of solution was the one that contained vinegar, so when test with a red litmus paper, it turns blue. The reaction takes place in less than a second, which makes the result quick and clear. It is also interesting because a colourless solution can change a colour strip into another colour, which is great because it will trigger the curiosity in people (if they don't know chemistry).
The next test was the solution that contains bleach, it changed the red strip into blue and the blue remained the same. Hence, the solution contains acid.
All in all, the results for today were :
1. Vinegar
- Turn blue > red
- Turn red > red
2. Bleach
-Turn red > blue
- Turn blue > blue
3. Baking soda
- Turn red > blue
- Turn blue > blue
However for some solutions, it turned the litmus paper into a total white strip over time.
Just a random thought and idea for the day, to use the petri dish as a display for the paper that went through the curiosity experiment and change of colour. I was thinking of displaying the beautiful stained paper using the petri dish and place them against the wall.
If I were to use the litmus paper test as part of the experiment, the instructions will be as follows :
1. Pick a bottle
2. Drip two drops on the paper
3. Pick up the red and blue litmus paper
4. Observe the colour change
5. Take away the litmus paper
6.Refer to chart for the cause in loss of curiosity
I am just worried that the implementation of the litmus paper will make the experiment for complicated for the audience because the current experiment is already a little too hard to understand by the audience. So there is still a lot to put into consideration!










No comments:
Post a Comment